Introduction to SNARE proteins
Eukaryotic cells contain membrane-bound compartments that are connected by trafficking of vesicular intermediates. To maintain compartmental organization, proper targeting of transport vesicles is required which involves two different molecular interactions.
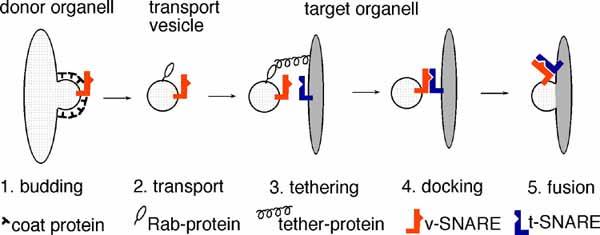
Membrane traffic is mediated by transport vesicles and can be subdivided into several steps

The first selective recognition of vesicle and target membrane takes place during docking. In this reaction specific rab/ypt small GTPases and tethering factors on opposite membranes interact. Docking then provides a signal for complex formation of specific evolutionary conserved transmembrane proteins that reside on vesicles (v-SNAREs) and target membranes (t-SNAREs). The acronyme SNARE means: soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor. 21 SNARE proteins have been found in yeast and over 35 in mammals so far. They are localized to specific organelles and take part in one or in a few membrane transport steps.
Binding of v- and t-SNAREs induces a conformational change and leads to membrane fusion
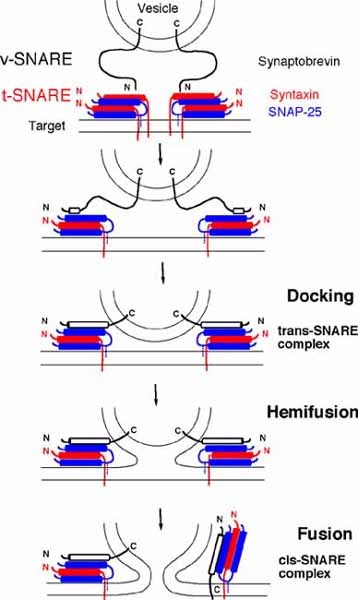
Conserved domains of v- and t-SNAREs close to the membrane anchor bind each other. Complex formation is accompanied by changes in the protein structure which probably drives membrane fusion. Recent structural studies suggest that SNARE complexes have a common structure: four conserved SNARE domains of about 50 amino acid residues form a parallel four-helix bundle. The helices interact through hydrophobic amino acid residues except in the center of these helices. Here an arginine (R) residue of a v-SNARE interacts with three glutamine (Q) residues from the other helices. These amino acids are very conserved leading to a reclassification of SNAREs as R- and Q-SNAREs. All t-SNAREs have a glutamine, while some v-SNAREs contain an arginine and others a glutamine in this 0 layer. Q-SNAREs can be subdivided into three different groups. According to an emerging model all SNARE complexes are formed by one helix from each of the four groups.
Alberts, B. et al.. (2002) Molecular Biology of the Cell. Fourth edition. Garland Publishing (Text book)
R. Jahn, T. Lang, T.C. Sudhof (2003) Membrane fusion. Cell 112, 519-533
J.B. Bock, H.T. Matern, A.A. Peden, R.H. Scheller (2001) A genomic perspective on membrane compartment organization. Nature 409, 839-841
H.R.B. Pelham (2001) SNAREs and the specificity of membrane fusion. TICB 11, 99-101