Nitrilases
Nitrilases catalyze the hydrolytic cleavage of nitriles (organic cyanides) to the corresponding carboxylic acids. Nitrilases of plants are divided
into two groups, depending if they are homologs nitrilase 4 (NIT4) or nitrilase 1 (NIT1) from Arabidopsis thaliana.
| NIT1 | NIT4 |
|---|---|
| restricted to Brassicaceae | distributed widely in the plant kingdom |
| broad substrate specificity | high substrate specificity for β-cyanoalanine |
| involved in glucosinolate catabolism(?) | involved in cyanide detoxification |
Project: Nitrilases involved in cyanide detoxification of higher plants
Plants may be challenged by the highly toxic compound cyanide (hydrocyanic acid) from two sources:
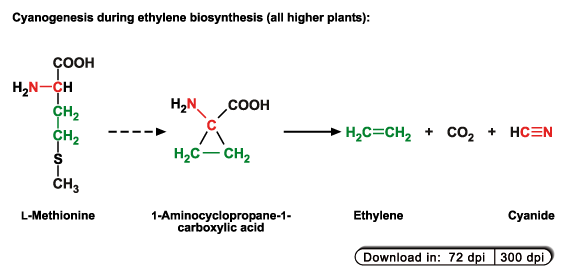
1. Biosynthesis of the plant hormone ethylene. Biosynthesis of this hormone is strongly induced e.g. by wounding, during senescence and
fruit ripening.
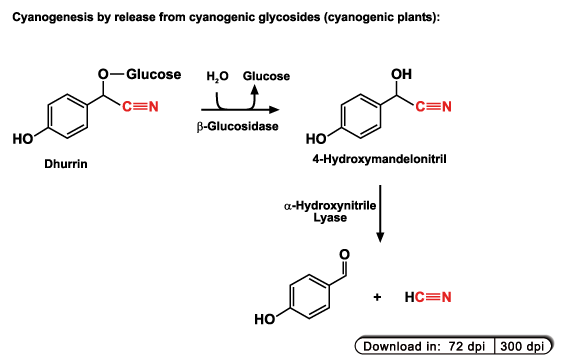
2. Wounding-induced breakdown of cyanogenic glycosides by the sequential action of β-glucosidases and α-hydroxynitrile lyases.
This process is restricted to plants which contain cyanogenic glycosides (approx. 2600 known species).
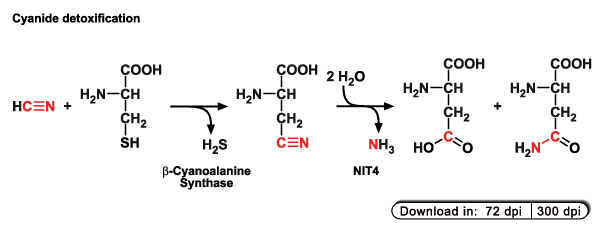
Plants are able to detoxify this cyanide via a two-step pathway. In the first step, cyanide is coupled to the amino acid L-cysteine,
thus forming the non-proteinogenic amino acid β-cyano-L-alanine. By this reaction the cyanide is detoxified but the formed
β-cyanoalanine is more or less useless for the plant. In the second step, therefore, the β-cyanoalanine is converted into the proteinogenic
amino acids L-asparagine and L-aspartic acid.
In the past we have shown that NIT4 of the model plant Arabidopsis thaliana and the NIT4 homologs of Nicotiana tabacum can use
β-cyanoalanine as substrate and that these enzymes form two products, namely asparagine and aspartic acid, at the same time
(Piotrowski et al., 2001). In addition we could show that the
β-cyanoalanine-hydrolyzing activity found in Lupinus angustifolius is also due to a NIT4 homolog. In contrast to the NIT4 enzymes
from A. thaliana and N. tabacum, the lupine NIT4 produces much more asparagine than aspartic acid (Volmer & Piotrowski, 2006).
Our current research on the NIT4 enzymes deals mostly with two aspects:
- As some plants contain more than one NIT4 homolog we wonder if the function of these enzymes is restricted to the metabolism of β-cyanoalanine or if they may fulfil additional functions in those plants.
- The typical reaction product of a nitrilase-catalyzed reaction is a carboxylic acid. What is the mechanism by which NIT4 enzymes can produce up to 4fold more amide (asparagine) than carboxylic acid?