Glucosinolate Metabolism
Glucosinolates are secondary compounds present in members of the order Brassicales (formerly Capparales). This order contains (among others)
the families Tropaeolaceae (e.g. nasturtiums), Caricaceae (e.g. papaya), Capparaceae (e.g. caper), Cleomaceae (e.g. spiny spider-flower) and Brassicacea
(including many vegetables like cabbage, cauliflower, Brussels sprouts, turnip, red and white radish, mustard, rapeseed, etc., and the model plant
Arabidopsis thaliana).
Glucosinolates are derived from proteinogenic amino acids (alanine, leucine, isoleucine, methionine, phenylalanine, tryptophan, tyrosin and valin), which
may undergo one to several rounds of chain elongation (especially methionine) before the glucosinolate core structure is synthesized. Most of the enzymes
involved in the core structure biosynthesis have been identified and cloned (for a recent review, see Halkier and Gershenzon, 2006).
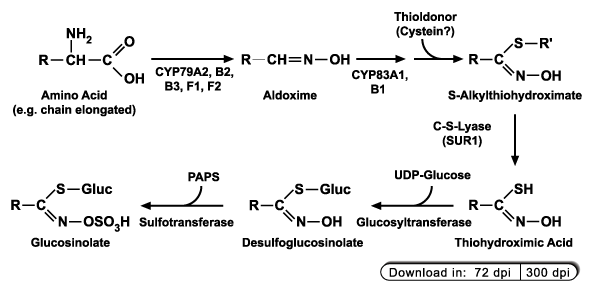
In the course of ongoing research in our department on coronatine-induced genes (Lopukhina et al., 2001)
we identified three PAPS:desulfoglucosinolate sulfotransferases catalyzing the last step in biosynthesis of the glucosinolate core structure (Piotrowski et al., 2004).
Glucosinolates are stored within the vacuoles of specialized cells. If the plants are wounded by the attack of a herbivore or a pathogen, the glucosinolates
will be released from the vacuole and came in contact with the thioglucosidase myrosinase. The intermediate product rearranges to form several possible products
depending on the reaction conditions, the glucosinolate side chain and the presence of certain protein cofactors like the epithiospecifier protein (ESP),
the thiocyanate forming protein (TFP) and the epithiospecifier modifier (ESM).
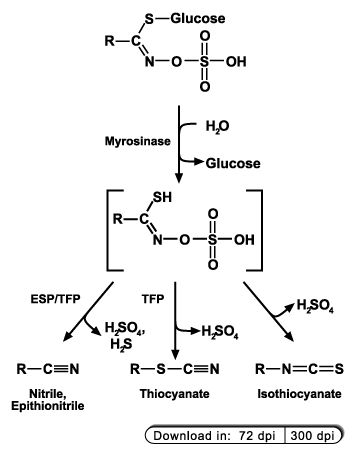
Because nitriles are among the possible breakdown products of glucosinolates we investigate the involvement of nitrilases in the process of glucosinolate catabolism.
In A. thaliana the two nitrilases NIT1 and NIT2 display broad substrate specificities and are able to convert glucosinolate-derived nitriles with high activity
(Vorwerk et al., 2001). In addition to the wounding-induced glucosinolate breakdown depicted
above, there are also numerous reports on an endogenous glucosinolate catabolism within plants of the Brassicaceae. We wonder if nitrilases of the NIT1 group (i.e. NIT1, NIT2,
NIT3, see also Research→Nitrilases) are part of this developmentally regulated glucosinolate catabolism.